Katie M. Horsman, Todd P. Chassee, and William F. Polik
Hope College, Holland, MI 49423, USA
Dispersed Fluorescence (DF) spectroscopy is used to measure the vibrational energy levels of formyl fluoride (HFCO) and thereby characterize its S0 potential energy surface. Sample molecules are cooled in a supersonic expansion and then excited to the 62 level of the S1 electronic state with 255nm light from a pulsed dye laser. Fluorescence is dispersed from 255nm to 600nm with a high resolution monochromator and detected with an intensified charge coupled device (ICCD). Analysis of the 22,500 cm-1 dispersed fluorescence spectrum results in 221 new assignments including 61 assignments above the approximate 17,000 cm-1 dissociation threshold. Primary progressions are observed in n2 (C=O stretch) and n6 (out-of-plane bend); however, progressions in n4 (C-F stretch) and n5 (FCO bend) are also evident. The data are fit to an anharmonic oscillator model with a standard deviation of 3.3 cm-1, resulting in a quantitative description of formyl fluoride as it undergoes unimolecular dissociation.
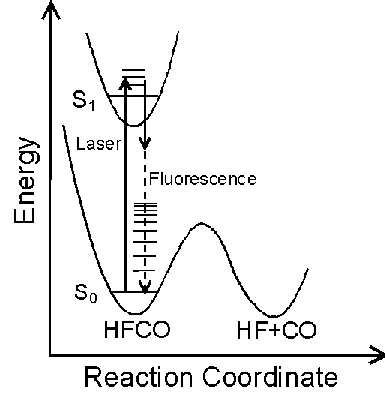
The Potential Energy Surface (PES) provides a fundamental description of a reaction mechanism. The PES of a molecule can be characterized by measuring its vibrational spectrum. Dispersed Fluorescence (DF) spectroscopy is a useful method for observing vibrational energy levels over a wide energy range.
HFCO has a lower dissociation energy (17,000 cm-1) than H2CO (28,000 cm-1) which was previously studied. Consequently, HFCO has detectable fluorescence to vibrational levels below, at, and above the dissociation threshold. This allows experimental characterization of the PES both below and above the activation energy of a chemical reaction.

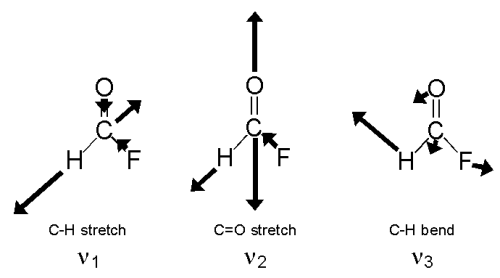
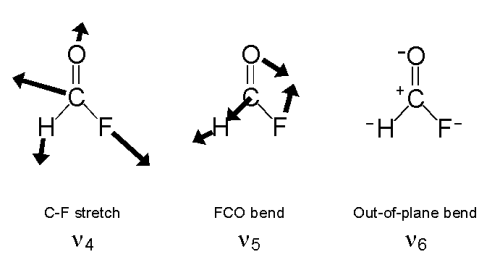
HFCO has six normal modes of vibration as shown below.
Light, molecules, and detection are the components of DF spectroscopy.
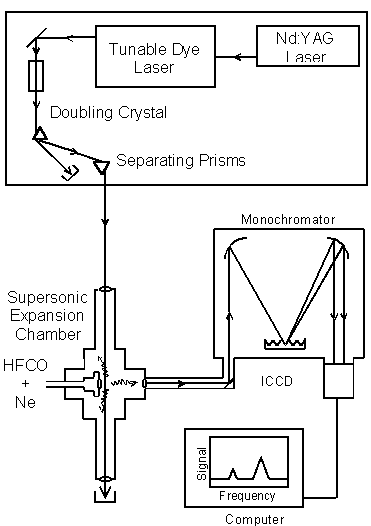
A Nd:YAG laser pumps a tunable dye laser. The laser light is converted to UV by the doubling crystal. A 60o prism separates the UV laser light from residual, undoubled light. The UV light is directed into the supersonic expansion chamber. HFCO molecules are seeded in neon, cooled in a supersonic expansion, and excited by the UV laser light. The fluorescence is imaged into a monochromator and dispersed onto an Intensified Charged Coupling Device (ICCD) detector. The DF spectrum is analyzed by a computer.
The frequency of the dye laser was tuned to the overlapped 101, 202, and 303 rotational levels in the S1 62 vibronic state. Excitation of Ka=0 levels result in a simplified dispersed fluorescence spectrum.
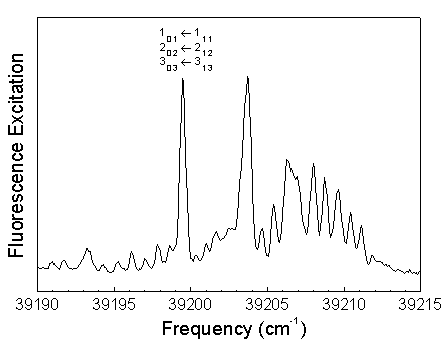
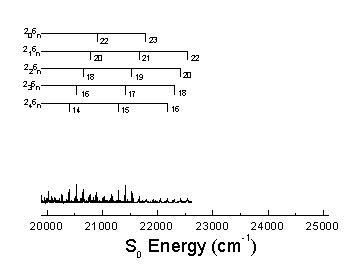
The S0 energy spectrum of HFCO fluorescing from the overlapped 101, 202, and 303 rotational levels in the S1 62 vibronic state is presented below. The spectrum extends far beyond the 0-4,000 cm-1 range of traditional infrared spectroscopy. Vibrational states are observed well above the 17,000 cm-1 dissociation threshold.
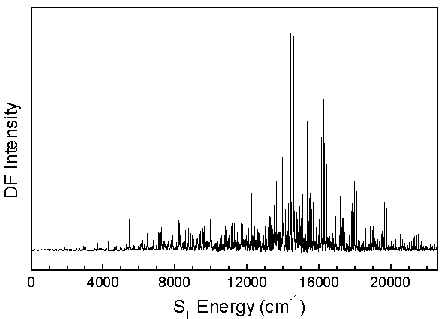
The n® p * transition results in a lengthening of the C=O bond and a geometry change from planar to pyramidal. Long progressions in n2 and n6, as indicated below, are evident due to the substantial geometry change in these normal modes. A total of 265 vibrational assignments were made, including short progressions in n4 and n5.
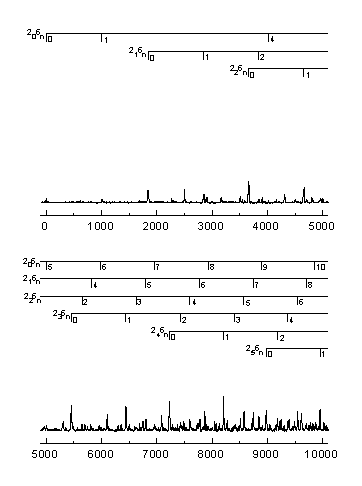
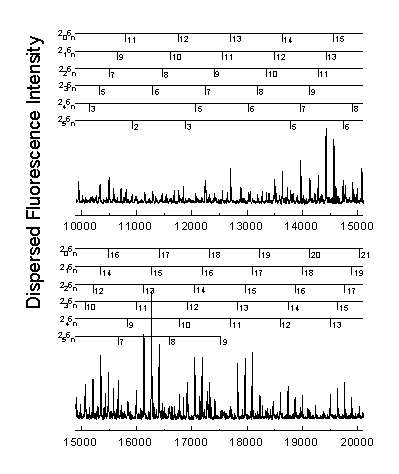
Assignments were made using the anharmonic oscillator model shown below. The increase in number of assignments allows the calculation of previously undetermined vibrational constants.
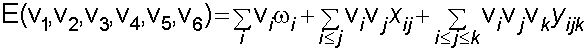
|
Parameter |
Choi et al.a (cm-1) |
Horsman et al. (cm-1) |
Theoryb (cm-1) |
|
w 1 |
3160.00 |
||
|
w 2 |
1848.79 |
1849.42 |
1845.00 |
|
w 3 |
1377.00 |
||
|
w 4 |
1071.51 |
1043.00 |
|
|
w 5 |
665.71 |
651.00 |
|
|
w 6 |
1013.87 |
1014.31 |
1027.00 |
|
x11 |
-60.61 |
||
|
x12 |
-1.20 |
||
|
x13 |
-21.19 |
||
|
x14 |
0.79 |
||
|
x15 |
0.64 |
||
|
x16 |
-15.62 |
||
|
x22 |
-12.00 |
-11.28 |
-10.81 |
|
x23 |
-4.59 |
||
|
x24 |
-4.41 |
-6.75 |
|
|
x25 |
-6.55 |
-4.79 |
|
|
x26 |
-7.24 |
-7.95 |
-7.34 |
|
x33 |
-8.90 |
||
|
x34 |
-8.79 |
||
|
x35 |
-1.34 |
||
|
x36 |
2.63 |
||
|
x44 |
-8.87 |
-6.77 |
|
|
x45 |
-8.88 |
-8.10 |
|
|
x46 |
-3.59 |
-3.93 |
|
|
x55 |
0.44 |
-0.59 |
|
|
x56 |
-0.49 |
-1.10 |
|
|
x66 |
-2.84 |
-2.84 |
-2.86 |
|
y222 |
0.06055 |
0.08442 |
|
|
y226 |
0.24218 |
0.15477 |
|
|
y266 |
-0.11770 |
-0.06836 |
|
|
y666 |
-0.00212 |
-0.00334 |
a. Y.S. Choi and C.B. Moore, J. Chem. Phys. 94, 5414 (1991)
b. W.H. Green et al., J. Chem. Phys. 93, 4965 (1990)
The vibrational spectrum of S0 HFCO has been recorded up to 22,500 cm-1 using dispersed fluorescence (DF) spectroscopy. A more complete assignment of the spectrum has resulted using an anharmonic oscillator model. The assignment of these excited vibrational states will be used to model the potential energy surface of HFCO including the important dissociation threshold region.
KMH gratefully acknowledges the Beckman Foundation and TPC gratefully acknowledges the Dreyfus Foundation for research fellowships. This research has been sponsored by the Wyckoff Chemical Company and National Science Foundation grant CHE-9157713.