Kevin W. Paulisse and William F. Polik
Hope College, Holland, MI 49423, USA
Acrolein is a prototypical conjugated organic molecule with interesting spectroscopic properties.

The S1 vibronic spectrum consists of transitions from vibrational levels of the ground electronic state to vibrational levels of the first excited singlet electronic state.
The acrolein vibronic spectrum was previously obtained in a molecular beam (70K) by cavity ringdown spectroscopy and at room temperature (295K) in a conventional spectrometer.
The spectrum is assigned by predicting the fundamental transitions using computational chemistry programs, analyzing Franck-Condon progressions, comparing spectra taken at different temperatures, and observing rotational band structure.
Assignment of the vibronic spectrum allows for a better understanding of the potential energy surface and photophysical properties of acrolein.
The Gaussian 94 program1 was used to predict the frequencies of the S1 normal modes.
Gaussian 94 was first used to calculate the S1 normal mode frequencies of formaldehyde (H2CO). Because the systems are similar, frequency scaling factors were computed from the H2CO calculations by comparing the results to experimental values from the literature.
Gaussian 94 was then used to calculate the S1 vibrational spectrum of acrolein using the RCIS method with two basis sets. All results are in cm-1 units.
| Mode | Description | 6-31+G(D) | 6-311+G(3DF,2PD) | Observed |
| 1 | Asym vinyl CH stretch | 3097 | 3055 | |
| 2 | Symm vinyl CH2 stretch | 3019 | 2975 | |
| 3 | Vinyl CH stretch | 3048 | 3006 | |
| 4 | Formyl CH stretch | 2896 | 2827 | |
| 5 | C=O stretch | 1180 | 1177 | 1262 |
| 6 | C=C stretch | 1432 | 1479 | 1406 |
| 7 | CH2 bend | 1406 | 1389 | |
| 8 | Formyl CH rock | 1157 | 1135 | 1132 |
| 9 | Vinyl CH rock | 1281 | 1267 | 1279 |
| 10 | Vinyl CH2 rock | 882 | 867 | |
| 11 | C-C stretch | 1070 | 1050 | |
| 12 | CCO bend | 471 | 468 | 483 |
| 13 | CCC bend | 301 | 301 | 302 |
| 14 | Vinyl C=C torsion | 611 | 613 | |
| 15 | Formyl CH wag | 378 | 393 | |
| 16 | Vinyl CH2 wag | 896 | 889 | 916 |
| 17 | Vinyl CH wag | 993 | 985 | |
| 18 | Skeletal torsion | 144 | 148 | 252 |
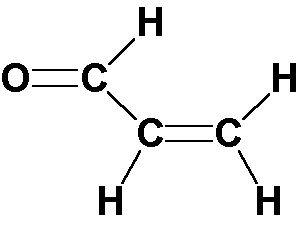
Franck-Condon progressions are series of peaks resulting from changes of a vibrational quantum number in a normal mode. The n18 mode shows a number of Franck-Condon progressions in the room temperature spectrum.
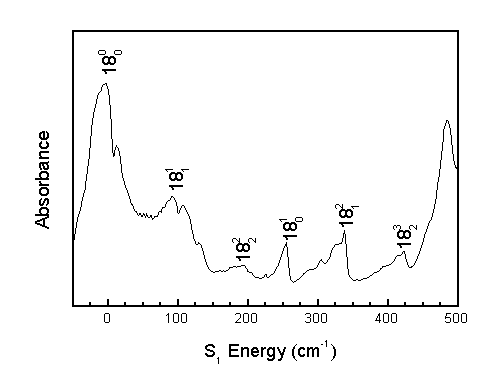
The Boltzmann factor predicts that only the vibrational levels of lowest energy will be populated at low temperatures. Consequently, if a band appears in the room temperature spectrum (295 K) but disappears in the molecular beam spectrum (70K), the corresponding transition must have originated from an excited vibrational energy level.
Transitions originating from ground vibrational levels are called "Cold Bands," while transitions originating from excited vibrational levels are called "Hot Bands."
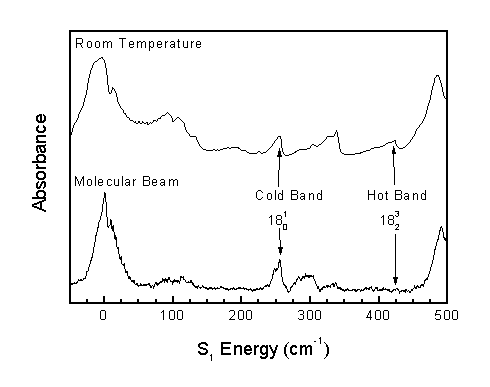
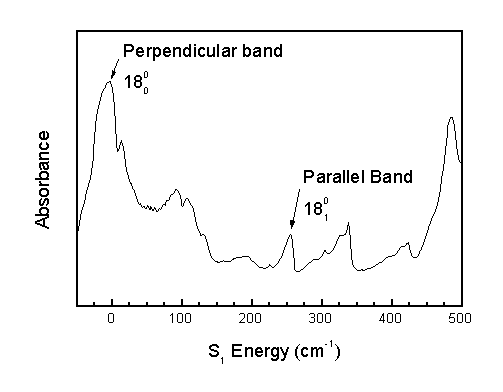
Two types of rotational band structures are observed in the acrolein spectrum. Narrow bands are called "parallel" bands, and broad bands are called "perpendicular" bands.
Parallel bands occur when the change in out-of-plane vibrational quanta (n14-n18) is odd. Perpendicular bands occur when the change in out-of-plane vibrational quanta is even.
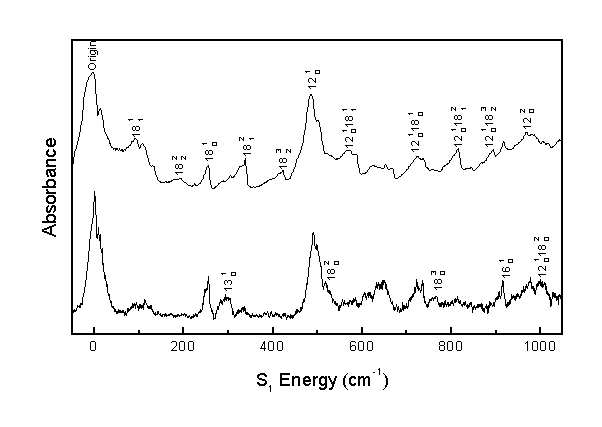
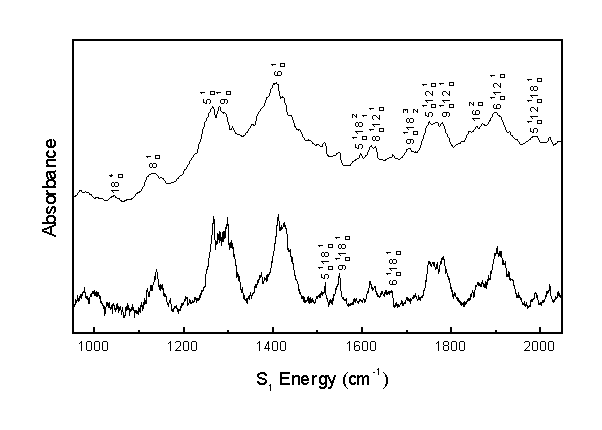
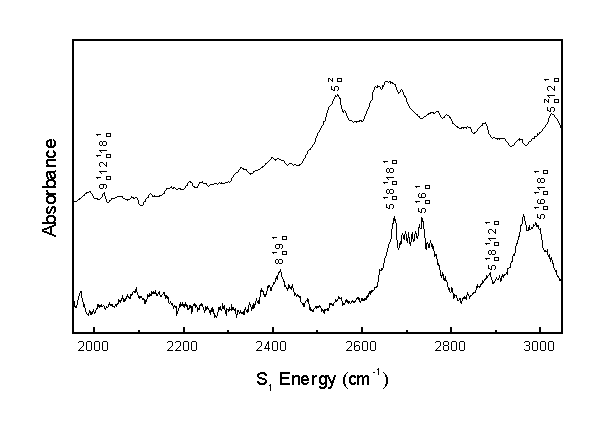
The following are the assignments for the acrolein S1 vibronic spectrum. All results are in cm-1 units. Calculated combination band frequencies are based on the observed fundamental frequencies.
| Assignment | Observed | Calculated |
| Origin | 0 | 0 |
| 18(1,1) | 91 | 98 |
| 18(2,2) | 192 | 196 |
| 18(1,0) | 254 | |
| 13(1,0) | 305 | 301 |
| 18(2,1) | 338 | 345 |
| 18(3,2) | 423 | 429 |
| 12(1,0) | 485 | |
| 18(2,0) | 517 | 508 |
| 12(1,0) 18(1,1) | 583 | 576 |
| 12(1,0) 18(1,0) | 724 | 739 |
| 18(3,0) | 763 | 762 |
| 12(1,0) 18(2,1) | 814 | 823 |
| 12(1,0) 18(3,2) | 893 | 908 |
| 16(1,0) | 916 | |
| 12(2,0) | 969 | 970 |
| 12(1,0) 18(2,0) | 997 | 1002 |
| 18(4,0) | 1043 | 1016 |
| 8(1,0) | 1138 | |
| 5(1,0) | 1263 | |
| 9(1,0) | 1279 | |
| 6(1,0) | 1407 | |
| 5(1,0) 18(1,0) | 1514 | 1517 |
| 9(1,0) 18(1,0) | 1545 | 1533 |
| 5(1,0) 18(2,1) | 1596 | 1591 |
| 8(1,0) 12(1,0) | 1619 | 1623 |
| 9(1,0) 18(2,1) | 1628 | 1617 |
| 6(1,0) 18(1,0) | 1667 | 1661 |
| 9(1,0) 18(3,2) | 1702 | 1702 |
| 5(1,0) 12(1,0) | 1750 | 1748 |
| 9(1,0) 12(1,0) | 1768 | 1764 |
| 6(1,0) 12(1,0) | 1902 | 1892 |
| 5(1,0) 12(1,0) 18(1,0) | 1988 | 2002 |
| 9(1,0) 12(1,0) 18(1,0) | 2018 | 2018 |
| 8(1,0) 9(1,0) | 2415 | 2417 |
| 5(2,0) | 2543 | 2526 |
| 5(1,0) 8(1,0) 18(1,0) | 2670 | 2655 |
| 5(1,0) 6(1,0) | 2734 | 2670 |
| 5(1,0) 8(1,0) 12(1,0) | 2886 | 2888 |
| 5(1,0) 6(1,0) 18(1,0) | 2988 | 2988 |
| 5(2,0) 12(1,0) | 3024 | 3032 |
The acrolein S1 vibronic spectrum was assigned considering the computed spectrum, Franck-Condon progressions, temperature effects, and rotational band structure. This allows for the correction of previous literature assignments and the identification of previously unassigned bands. This assignment identifies 41 bands, whereas previous literature2 identified 21 bands.
The computation of the spectrum allows for assignment of the 9(1,0) and 13(1,0) fundamental transitions and validates the assignment of the other fundamental transitions.
Comparison of the spectrum obtained at room temperature to the spectrum obtained in a molecular beam allows the correction of previous literature assignments.2 The previous assignments of 14(1,0) and 16(1,0) corresponded to hot bands, and the previous assignment of 15(1,0) corresponded to a perpendicular band when, in actuality, 15(1,0) must be a parallel band.
The band identified as 14(1,0) by the previous assignment is actually 18(2,1). Consequently, many more combination bands involving n18 are observed than previously believed, allowing for a better understanding of the S1n18 potential energy surface.
The observed bands in the room temperature and molecular beam spectra were assigned as shown. Assignments over 2000 cm-1 are tentative.
We gratefully acknowledge Tyson O. Friday and Margaret L. Graske for recording the S1 acrolein vibronic spectra. This research has been sponsored by National Science Foundation grant CHE-9157713.
1 Gaussian 94, Revision E.2, M. J. Frisch, G. W. Trucks, H. B. Schlegel, P. M. W. Gill, B. G. Johnson, M. A. Robb, J. R. Cheeseman, T. Keith, G. A. Petersson, J. A. Montgomery, K. Raghavachari, M. A. Al-Laham, V. G. Zakrzewski, J. V. Ortiz, J. B. Foresman, J. Cioslowski, B. B. Stefanov, A. Nanayakkara, M. Challacombe, C. Y. Peng, P. Y. Ayala, W. Chen, M. W. Wong, J. L. Andres, E. S. Replogle, R. Gomperts, R. L. Martin, D. J. Fox, J. S. Binkley, D. J. Defrees, J. Baker, J. P. Stewart, M. Head-Gordon, C. Gonzalez, and J. A. Pople, Gaussian, Inc., Pittsburgh PA, 1995.
2J.D.C. Brand, D.G. Williamson, Discussions Faraday Soc. 35 (1963) 184