J. Ruud van Ommen and William F. Polik
Hope College, Holland, MI 49423, USA
http://www.chem.hope.edu/~polik/poster/polyad96.htm
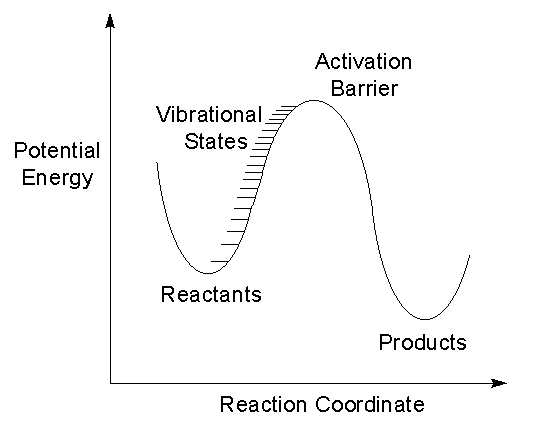
Reacting molecules must be in highly excited states to have the energy necessary to overcome the activation barrier and form products. Spectroscopic study of highly excited molecules will help chemists understand reacting molecules.

The potential energy surface of a molecule can be experimentally characterized by measuring vibrational states. A model which describes the observed vibrational state structure is useful in assessing potential energy surface calculations.
The formaldehyde molecule (H2CO) has six different vibrational normal modes.
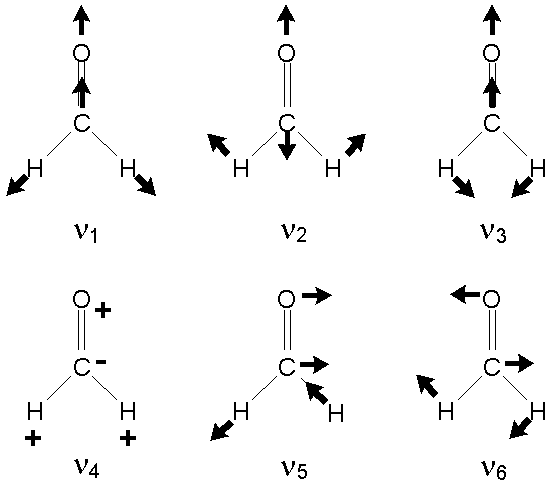
The molecule can have one or more quanta of energy in each mode. The number of quanta in mode i is denoted by vi. An example of the notation used to represent a vibrational state is 215164 for a state with v2=1, v5=1, and v6=4.
In previous experiments by Bouwens et al. [J. Chem. Phys. 104, 460 (1996)], energy values of 270 vibrational states were obtained by dispersed fluorescence spectroscopy. In this poster, a model for those states is presented.
The energy of the vibrational levels can be described by the anharmonic model. This model includes a harmonic frequency w0i for each mode and an anharmonic correction xij for each pair of modes. Accordingly, we can describe the energy of a vibrational level as follows:

However, the higher the energy, the worse the predictions of this model. This inadequacy is due to interactions between different states that are not taken into account.
States close in energy can interact by a resonance. For example, the k26,5 resonance mixes a quantum in mode 2 and a quantum in mode 6 with a quantum in mode 5:

By including more resonances in the model, larger and more complex sets of connected states are formed:

A set of states connected by resonances is called a polyad. The polyad model describes energies of connected states using a matrix. All non-resonant interactions are treated by second order perturbation theory yielding diagonal matrix elements similar to the anharmonic model:

All resonant interactions are treated explicitly yielding off-diagonal matrix elements. The matrix elements are of the form:

The vibrational energy levels are calculated by diagonalizing the resulting matrix.
The polyad model with different sets of resonances has been fitted to the experimental data up to 7000 cm-1 and up to 10000 cm-1. The standard deviations of the fits are given in the following table.
| Fit 1 | Fit 2 | Fit 3 | Fit 4 | Fit 5 | Fit 6 | Fit 7 | |
|---|---|---|---|---|---|---|---|
| k26,5 | x | x | x | x | x | x | |
| k36,5 | x | x | x | x | x | x | |
| k11,55 | x | x | x | x | x | x | |
| k44,66 | x | x | x | x | x | ||
| k25,35 | x | x | x | x | |||
| k26,36 | x | x | x | ||||
| k1,44 | x | x | |||||
| k1,66 | x | ||||||
| 0–7000 cm-1 fit std. dev. (cm-1) | 11.19 | 3.16 | 2.64 | 2.57 | 2.39 | 2.39 | 2.23 |
| 0–10000 cm-1 fit std. dev. (cm-1) | 23.39 | 4.34 | 3.34 | 2.80 | 2.76 | 2.62 | 2.57 |
The parameters obtained by fitting the experimental values in the range 0–10000 cm-1 are given below.
| Parameter (cm-1) | Fit 1 | Fit 2 | Fit 3 | Fit 4 | Fit 5 | Fit 6 | Fit 7 |
|---|---|---|---|---|---|---|---|
| w01 | 2818.9 | 2812.3 | 2813.7 | 2817.4 | 2818.0 | 2815.5 | 2809.9 |
| w02 | 1759.9 | 1755.6 | 1755.8 | 1755.7 | 1755.8 | 1756.0 | 1756.0 |
| w03 | 1479.5 | 1499.1 | 1499.3 | 1499.2 | 1498.8 | 1499.1 | 1499.4 |
| w04 | 1169.8 | 1170.5 | 1169.8 | 1169.7 | 1169.7 | 1167.3 | 1167.3 |
| w05 | 2859.6 | 2882.8 | 2879.2 | 2873.8 | 2875.9 | 2876.0 | 2877.7 |
| w06 | 1260.6 | 1254.8 | 1251.5 | 1251.9 | 1251.8 | 1251.9 | 1249.8 |
| x11 | -40.1 | -29.8 | -30.7 | -34.4 | -35.0 | -35.9 | -33.8 |
| x12 | 1.3 | 0.9 | 0.7 | 0.4 | 0.4 | 0.3 | 0.5 |
| x13 | -35.5 | -27.2 | -27.7 | -26.9 | -26.8 | -27.2 | -27.6 |
| x14 | -8.5 | -9.3 | -9.5 | -9.4 | -9.4 | -15.0 | -14.9 |
| x15 | -127.3 | -133.9 | -130.8 | -131.5 | -133.4 | -136.1 | -137.0 |
| x16 | -3.2 | -12.2 | -12.8 | -13.2 | -13.0 | -13.4 | -17.6 |
| x22 | -10.6 | -10.1 | -10.1 | -9.9 | -9.9 | -9.9 | -9.9 |
| x23 | -4.8 | -7.0 | -7.1 | -8.0 | -7.8 | -7.9 | -8.1 |
| x24 | -7.4 | -6.8 | -6.8 | -7.0 | -7.1 | -7.2 | -7.3 |
| x25 | -23.4 | 5.5 | 5.2 | 1.7 | 1.4 | 1.2 | 0.9 |
| x26 | -0.8 | -15.5 | -14.2 | -11.6 | -12.2 | -12.2 | -12.1 |
| x33 | 10.8 | 0.3 | 0.4 | 0.6 | 0.6 | 0.5 | 0.5 |
| x34 | -2.7 | -1.9 | -1.5 | -1.4 | -1.4 | -1.1 | -1.2 |
| x35 | -3.8 | -17.3 | -17.2 | -23.9 | -23.9 | -23.4 | -21.1 |
| x36 | -23.3 | -12.9 | -12.4 | -8.7 | -8.0 | -8.6 | -9.2 |
| x44 | -3.0 | -3.2 | -2.9 | -2.8 | -2.8 | -0.9 | -0.9 |
| x45 | -13.5 | -22.2 | -21.8 | -22.0 | -22.2 | -20.6 | -20.2 |
| x46 | 1.7 | 3.1 | 5.2 | 5.3 | 5.5 | 5.6 | 5.5 |
| x55 | -14.3 | -38.2 | -36.3 | -34.7 | -35.5 | -36.0 | -37.6 |
| x56 | -9.8 | -0.2 | -3.0 | -13.5 | -14.4 | -13.9 | -12.2 |
| x66 | -5.2 | -2.8 | -2.1 | -2.2 | -2.3 | -2.2 | -0.7 |
| k26,5 | 148.6 | 146.7 | 138.6 | 143.7 | 143.6 | 145.1 | |
| k36,5 | 129.3 | 129.6 | 135.1 | 130.9 | 130.7 | 129.3 | |
| k11,55 | -140.5 | -137.4 | -129.3 | -127.8 | -132.4 | -138.2 | |
| k44,66 | -21.6 | -23.3 | -24.2 | -23.4 | -18.1 | ||
| k25,35 | -18.5 | -23.1 | -23.1 | -23.2 | |||
| k26,36 | -5.7 | -5.7 | -6.9 | ||||
| k1,44 | 78.0 | 79.4 | |||||
| k1,66 | 55.0 |
The first table shows that the fit improves by increasing the number of resonances, but with diminishing returns. A good fit can be obtained with only a few resonances; the fit standard deviation of about 3 cm-1 compares well to the experimental standard deviation of 1 cm-1.
For fitting the 0–7000 cm-1 data, using the first four resonances (Fit 3) seems to be sufficient to obtain a good fit. The vibrational states up to 10000 cm-1 are fit well by including the first five resonances (Fit 4).
The second table shows that including more resonances affects the value of previous resonance parameters only slightly. This demonstrates that the parameters determined are relatively independent of the resonances included.
The polyad model gives a good description of experimentally obtained values of formaldehyde vibrational states. The key resonances are determined for formaldehyde. By including only five resonances, states up to 10000 cm-1 can be fit with a standard deviation of only 2.8 cm-1.
We gratefully acknowledge Rychard J. Bouwens for assigning the H2CO vibrational states. In addition, we express thanks to Dr. Robert W. Field, Dr. Anne B. McCoy, and Dr. Edwin L. Sibert III for valuable discussions. This research has been sponsored by National Science Foundation grant CHE-9157713 and the 1996 Summer Research Grant Award of the Royal Netherlands Chemical Society, Division of Chemical Education.