Assignment
CompChem7: Applications III
1. UV-Vis Spectra of Conjugated Aldehydes
Build and perform Geometry Optimization calculations for the following molecules. Using the resulting geometry, perform UV-Vis Spectra calculations for each molecule.
|
molecule |
2-propenal |
2-butenal |
2,4-pentadienal |
|
Abs Max (nm) |
209 |
221 |
251 |
The first excited state (A²) corresponds to a forbidden n®p* transition and is therefore extremely weak. The second excited state (A¢) corresponds to an allowed p®p* transition and is strongly absorbing. Compare the computed absorption maximum to the experimentally observed maximum in a table with columns for molecule, computed maximum, experimental maximum, and difference. Comment on any trends that are observed.
2. Excited State Optimization and Vibrational Frequencies of Formaldehyde (Gaussian)
Build ground state (S0) formaldehyde, H2CO, and perform a Hartree-Fock 6-31G(d) Geometry Optimization calculation. Using the resulting geometry, perform a Vibrational Frequencies calculation at the same level of theory.
The first excited singlet state (S1) of formaldehyde is pyramidal. Build S1 formaldehyde, and move the O atom out of the molecular plane by adjusting the O-H-H-C dihedral angle to 20 degrees. Perform a Hartree-Fock 6-31G(d) Geometry Optimization, but use the Advanced Options dialog box to add the Additional Keyword “CIS”. Using the resulting geometry, perform a Vibrational Frequencies calculation at the same level of theory and with the Additional Keyword “CIS”.
Complete the following table, use the scaling factor of 0.8929 for Hartree-Fock 6-31G(d) frequency calculations.
|
|
|
S0
Scaled |
S1
Scaled |
S1 Literature
|
|
n1 |
sym CH stretch |
|
|
2846 |
|
n2 |
CO stretch |
|
|
1183 |
|
n3 |
CH2 bend |
|
|
1293 |
|
n4 |
out-of-plane bend |
|
|
-- |
|
n5 |
antisym CH stretch |
|
|
2968 |
|
n6 |
CH2 rock |
|
|
904 |
Which vibrational mode undergoes the largest change upon electronic excitation? Offer an explanation for your result, noting that the S0®S1 eletronic transition is an n®p* transition.
3. Solvation Energies of Cations.
Build the ammonium cation, NH4+, and perform a Geometry Optimization calculation. Be sure to set the charge appropriately for a cation. Using the resulting geometry, perform another Geometry Optimization calculation, but use the Advanced Options to select water as the solvent.
Repeat the gas phase and aqueous calculations for tetramethyl ammonium cation, N(CH3)4+.
In WebMO Pro, select the four jobs in Job Manager and click the Spreadsheet button to obtain an immediate comparison among the four calculations.
Solvation energy is the energy change upon solvation, i.e., Esolvation = Esolution - Egas. The experimental solvation energies for NH4+ and C(CH3)4+ are -88 kcal/mol and -60 kcal/mol, respectively. Make a table with columns for molecule, gas phase energy, solution energy, calculated solvation energy, and experimental solvation energy. Comment on the accuracy of the solvation model being used.
4. Solvation Energy of Glycine and its Zwitter Ion
The amino acid glycine has a zwitter ion isomer, in which the carboxylic acid proton is transferred to the amino group.

Build glycine and perform a Geometry Optimization calculation. Using the resulting geometry, perform a Molecular Energy calculation at the same level of theory but using the Advanced Options to select water as the solvent.
Repeat the gas phase Geometry Optimization and aqueous Molecular Energy calculations for the zwitter ion.
In WebMO Pro, select the four jobs in Job Manager and click the Spreadsheet button to obtain an immediate comparison among the four calculations.
Solvation energy is the energy change upon solvation, i.e., Esolvation = Esolution - Egas. Make a table with columns for molecule, gas phase energy, solution energy, and solvation energy.
Which isomer is more stable in the gas phase? Which isomer is more stable in water? Comment on the reason for the difference in solvation energies between the two isomers.
5. Electrostatic Potential Surface of Imidazole (WebMO Pro)
Imidazole has two basic sites on the nitrogen atoms. The more basic site can be determined by locating the region of negative charge density around the molecule.
Build imidazole, C3H4N2,
and optimize the geometry with a PM3 calculation. View the result, choose New Job Using This Geometry, and perform
a Molecular Orbitals calculation at the same level of theory. On the job results page, view the
Electrostatic Potential, which opens the MOViewer application. The electrostatic potential is painted onto
an electron density isosurface, with red being negative and blue being
positive. In Edit:Preferences...,
adjust the Opacity of the surface to about 70%.
Use the electrostatic potential image to determine which nitrogen is more basic, i.e., would more likely attract a proton.
Confirm your prediction by computing the energies of the NH protonated cation and for the N protonated cation.
6. Nucleophilic (LUMO) Frontier Density (WebMO Pro)
Carbonyl groups undergo nucleophilic attack, which could in principle occur at either the carbon or at the oxygen atom. The nucleophilic attack site is a region where the nucleophile can donate its electron density to the carbonyl group. The site and orientation of nucleophilic attack can be inferred by viewing the carbonyl group LUMO, or directly viewed by visualizaing the LUMO density.
Build acetone, C3H6O, and optimize the geometry with a Hartree-Fock STO-3G calculation. View the result, choose New Job Using This Geometry, and perform a Molecular Orbitals calculation at the same level of theory.
On the job results page, view the LUMO (Lowest Unoccupied Molecular Orbital), which opens the MOViewer application. Infer whether the largest contribution comes from the carbonyl carbon or carbonyl oxygen.
View the Nucleophilic (LUMO) Frontier Density. The Frontier Density is painted onto an electron density isosurface, with red being smallest and blue being the largest. In Edit:Preferences..., adjust the Opacity of the surface to about 70%. Which atom is most likely to be attacked by a nucleophile? Is the attack likely to come from in the plane of the carbonyl group, or out of the plane?
7. Electrophilic (HOMO) Frontier Density (WebMO Pro)
Enolate anions undergo electrophilic attack, which can occur at either the oxygen atom or at the beta-carbon atom.
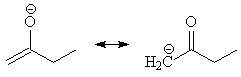
Build ethyl enolate, by first building the corresponding alcohol and then deleting the alcohol H atom. Optimize the geometry with a Hartree-Fock STO-3G calculation. View the result, choose New Job Using This Geometry, and perform a Molecular Orbitals calculation at the same level of theory.
On the job results page, view the HOMO (Highest Occupied Molecular Orbital), which opens the MOViewer application. Note that the electron density is delocalized across oxygen-carbon p system.
View the Eletrophilic (HOMO) Frontier Density. The Frontier Density is painted onto an electron density isosurface, with red being smallest and blue being the largest. In Edit:Preferences..., adjust the Opacity of the surface to about 70%. Which atom is most likely to be attacked by an electrophile
8. A new version of WebMO is about to be released, incorporating many of your previous comments. There is time for just one last set of suggestions before we stop modifying the code. Please make your final suggestions, particularly regarding MOViewer and other advanced features.