|
|
|
|
| Example: |
A + 2B ® 3C + D
|
Observe that use of the stoichiometric coefficient insures that the rate of a chemical reaction is the same regardless of which chemical species is used in the definition. In the above example, 1 molecule of A is consumed for every 3 molecules of C created; however, the reaction rates are equal due to inclusion of the stoichiometric coefficient.
where k is a rate constant, m is reaction order with respect to A, n is the reaction order with respect to B, and m+n is the overall reaction order.
The rate constant is independent of the concentrations of the chemical species involved in the reaction. However, it depends on other factors such as temperature or ionic strength, e.g., k(T).
The units of the rate constant depend on the overall reaction
order. The can be deduced by recognizing that Rate has units of concentration
per time. Concentration is usually measured in molarity (M) for solution
reactions and in molecules/cm3 for gas phase reactions.
| Example: | 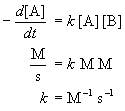 |
| Example: | Br2 + I2 ®
2BrI which reacts by a one-step mechanism
|
| Example: | Br2 + H2 ®
2HBr which reacts in a complicated mechanism in which the product, HBr,
can react with a reactant, Br2.
|
is known to be acid-catalyzed. Thus, the following form of the rate law is assumed
Bromine is a reddish-brown, and all the other reactants and products are clear. Thus, the reaction rate is conveniently measured by monitoring the concentration of bromine with a spectrophotometer.
The form of the rate law can be simplified by arranging the experimental conditions so that the concentration of one species is much smaller than the others, a technique known as "flooding". The concentration of the other species therefore remain essentially constant during the course of the reaction.
In this case, [Br2] << [CH3COCH3], [H+]. Thus the rate law reduces to
where
The exponent q can be determined by measuring the concentration of bromine [Br2] as a function of time t and comparing the observed data to the integrated rate laws for various values of q:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The rate constant k is then determined for each reaction run through the definition of kobs.
| Example: | 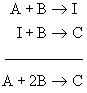 |
The bromination of acetone is a reaction for which the first step is believed to be the rate limiting step. Thus, the form of the rate law predicts the first step of the reaction mechanism.
It is generally true that the molecularity of the transition state can be determined from the form of the rate law. See Espenson, "Chemical Kinetics and Reaction Mechanism", McGraw-Hill, 1981, p. 90 for a very readable discussion on this topic.
Note that it is usually the case that the rate law is known from experiment and that the elementary steps must be postulated so as to be consistent with the observed rate law. It is for this reason that it is not possible to "prove" a mechanism using kinetics. Rather, reasonable mechanisms are put forward, and kinetic experiments are designed to distinguish among them.
|
|
The values of A and Ea can be determined by linearizing the Arrhenius equation
A plot of ln k versus 1/T will have a slope of -Ea/R and an intercept of ln A.
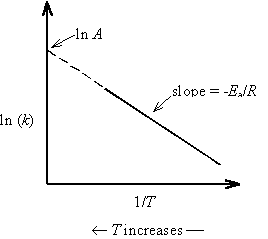
In the collision theory of bi-molecular gas phase reactions, the frequency factor A is interpreted as the frequency of encounters among reactants irrespective of energy. The activation energy Ea is the minimum energy reactants must possess in order to form products. The Boltzmann distribution predicts that the exponential function in the Arrhenius equation is the fraction of collisions with energy greater than Ea. The product of the frequency factor and the Boltzmann exponential functions therefore gives the reaction rate constant. There is an analogous interpretation of these terms for reactions that occur in solution.
In light of the collision theory interpretation, the activation energy Ea is also often interpreted as the energy of the transition state relative to the reactants.
Collision theory is the most simple model of chemical kinetics. Reactions involving free radicals or stable intermediates are known to display Arrhenius plots with negative values of Ea! The interpretation of activation energy as the energy of the transition state is not applicable in these cases, as the actual kinetics is much more complex than the collision theory model.