Principles of NMR Spectroscopy
1. What is NMR Spectroscopy?
NMR is an acronym for Nuclear Magnetic Resonance. NMR spectroscopy is a powerful tool for identifying nuclei based on the interaction of electromagnetic fields with a sample in a magnetic field. The technique has developed from an interesting physical curiosity in the 1940’s into one of the most important methods of spectral identification in chemistry, biochemistry, and medicine.
The goals of these notes are to provide an intuitive understanding of the NMR phenomenon. Underlying concepts will be emphasized over mathematical formalism.
2. Background
2.1 Classical Angular Momentum
Just as linear momentum
represents the tendency of an object move in a straight line, angular momentum
represents the tendency of an object to move in angular motion or rotate. Since momentum has both a magnitude and a
direction, it is a vector quantity.
Linear momentum is represented by a vector in the direction of
motion. Angular momentum, however, is
represented through use of the Right Hand Rule (RHR): when the fingers of the
right hand are curled in the direction of circular motion, the thumb points in
the direction of the angular momentum vector.
Mathematically, this is because the angular momentum vector ![]() is the cross-product
of the position
is the cross-product
of the position ![]() and linear momentum
and linear momentum ![]() vectors
vectors
![]() (1)
(1)
There are two kinds of classical angular momentum: orbital angular momentum and spin angular momentum. Orbital angular momentum is the circular motion of an object about a point, like the earth orbiting about the sun once a year. Spin angular momentum is the spinning motion of an object about its own axis, like the earth spinning about its axis once a day.
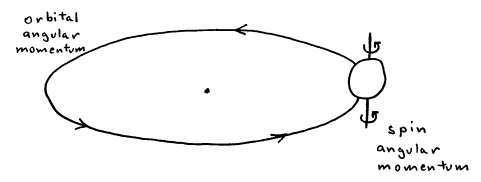
Figure 1. Orbital vs. spin angular momentum.
Exercise 1: Where do the angular momentum vectors lie for each case in Figure 1?
2.2 Quantum Mechanical Angular Momentum
Just as energy is known to be quantized at the atomic level, angular momentum is quantized. For example, an electron in an atom may only have orbital angular momentum quantum numbers l = 0, 1, 2, ... (which are more commonly denoted s, p, d, ...) and orbital magnetic quantum numbers ml = -l, -l+1, ..., l (which lead to px, py, and pz, for l=1 and to the five d orbitals for l=2). Also, the electron only has spin angular momentum quantum number s = 1/2 and spin magnetic quantum numbers ms = -1/2, 1/2 (which are also known as spin down and spin up).
All types of angular momentum
obey the same quantum mechanical rules.
In quantum mechanics, an angular momentum vector ![]() is restricted to
having a magnitude of
is restricted to
having a magnitude of
where
L = 0, 1/2, 1, 3/2, ... (3)
is the angular momentum quantum
number and
![]() = h/2p (4)
= h/2p (4)
is called
“h-bar” and equals Planck’s constant divided by 2p. One
component of the angular momentum vector, which is conventionally chosen to be
the z‑component,
is restricted to having values of
![]() (5)
(5)
where
mL = -L, -L+1, ..., L (6)
is the
magnetic quantum number.
Since the
magnitude of the angular momentum vector is greater than its maximum projection
on the z‑axis
![]() (7)
(7)
it is not
possible for ![]() to lie along the z‑axis. Rather
to lie along the z‑axis. Rather ![]() is always tipped away from its quantization
axis.
is always tipped away from its quantization
axis.
A
consequence of the Heisenberg Uncertainty Principle is that only one component
of angular momentum can be precisely specified. Defining the z‑component of angular momentum
results in the x‑ and y‑components being not well-defined,
and the direction in which ![]() tips away from the z‑axis is therefore
not known. It can be shown that the
expectation, or average, values of the x‑ and y‑components of
angular momentum are each zero
tips away from the z‑axis is therefore
not known. It can be shown that the
expectation, or average, values of the x‑ and y‑components of
angular momentum are each zero
![]() (8)
(8)
A useful
picture for representing the different angular momentum quantum states is to
imagine 2L+1
vectors with the same quantized magnitudes of ![]() but different
quantized projections on the z-axis of
but different
quantized projections on the z-axis of ![]() and undefined
projections on the x‑ and y‑axes.
and undefined
projections on the x‑ and y‑axes.
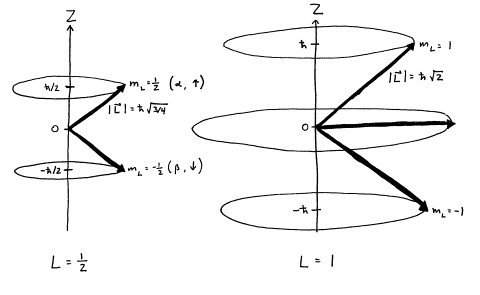
Figure 2. Quantized
angular momentum vectors for L=1/2 and L=1.
Exercise 2: Calculate the angle that the L=1/2,
mL=1/2
state lies away from the z‑axis. (Hint: Recall that “cosine equals adjacent over hypotenuse” for a
right triangle.)
2.3 Nuclear Spin
Spin is a fundamental property of particles, analogous to mass and charge. It is the angular momentum that is intrinsic to the particle, rather than the angular momentum arising from the overall motion of the particle in space. The spin quantum number I of a nucleus depends on the nuclear species, and has been observed to follow the pattern in Table 1.
|
Mass Number |
Atomic Number |
Nuclear Spin (I) |
Example |
|
odd |
even or odd |
1/2, 3/2, 5/2, ... |
I(1H) = 1/2 |
|
even |
even |
0 |
I(12C) = 0 |
|
even |
odd |
1, 2, 3, ... |
I(2H) = 1 |
Table 1. Nuclear Spin Quantum Numbers.
Exercise 3: Observation of an NMR spectrum requires that a nucleus have nonzero spin. Explain why carbon NMR spectra are weak, but still observable. (Hint: The molar mass of natural carbon is 12.011 rather than 12.000.)
Attempts to rationalize the existence of nuclear spin have been made using the model of a spinning charged particle. Classically, it is known that a moving electric charge induces a magnetic field. Since the nucleus has finite diameter and a positive charge, it would generate a magnetic field as it spins about its axis. However, a quantitative analysis of this model yields a value for the intrinsic magnetic field about an electron twice as large as it should be (which is accounted for by the Landé g factor), and the model cannot explain the observation that the spin of an electrically neutral neutron is 1/2 (see the Table 1). While the picture of a spinning nucleus is classically appealing, spin angular momentum is best treated as a quantum mechanical phenomenon.

Figure 3. Classical model of nuclear spin.
2.4 Nuclear Magnetic Moment
The nuclear spin quantum number
I
gives rise to nuclear spin angular momentum ![]() . The nuclear spin
angular momentum in turn gives rise to a nuclear magnetic moment
. The nuclear spin
angular momentum in turn gives rise to a nuclear magnetic moment ![]() according to
according to
![]() (9)
(9)
where g is the gyromagnetic ratio (or more correctly the magnetogyric ratio, whose name originates as the ratio of the “magneto” = “magnetic object” over the “gyric” = “turning object”). Each nuclear species has a different value of g, which is experimentally determined.
Since the nuclear magnetic moment vector is directly proportional to the nuclear spin angular momentum vector, the nuclear magnetic moment obeys the same rules of quantization as angular momentum,
![]() (10)
(10)
and
![]() (11)
(11)
where
mI = -I, -I+1, ..., I (12)
Exercise 4: Draw a picture of nuclear magnetic moment vectors for I=3/2 analogous to the picture of spin angular momentum vectors in Figure 2. Label the magnitude and z‑projection of each vector.
|
|
|
Natural Abundance |
Magnetogyric Ratio (g) |
Relative Frequency (n) |
|
1H |
1/2 |
99.985 |
26.752196 |
100.00 |
|
2H |
1 |
0.015 |
4.106625 |
15.35 |
|
13C |
1/2 |
1.10 |
6.72828 |
25.15 |
|
15N |
1/2 |
0.366 |
-2.712621 |
10.14 |
|
17O |
5/2 |
0.037 |
-3.62808 |
13.56 |
|
19F |
1/2 |
100.0 |
25.18147 |
94.13 |
|
29Si |
1/2 |
4.67 |
-5.319 |
19.88 |
|
31P |
1/2 |
100.0 |
10.8394 |
40.52 |
|
119Sn |
1/2 |
8.58 |
-10.0318 |
37.27 |
Table 2. Spin, natural abundance, magnetogyric ratio, and relative frequency for some common NMR nuclei.
Exercise 5: Calculate mz for a single proton with mI=1/2 in SI units. (Hint: The quantum number I is unitless, and J = kg·m2·s-2.) [Answer: mz = 1.4106´10-26 m2·A]
3. Effect of a Magnetic Field
3.1 Nuclear Energy Levels
In the absence of external
fields, there is no preferred orientation for a magnetic moment. In the presence of a magnetic field ![]() , however, the energy of a magnetic moment
, however, the energy of a magnetic moment ![]() depends on its
orientation relative to the field lines.
Classically,
depends on its
orientation relative to the field lines.
Classically,
![]() (13)
(13)
The energy is a minimum when the magnetic moment is aligned parallel to the magnetic field and a maximum when it is anti-parallel to the magnetic field.
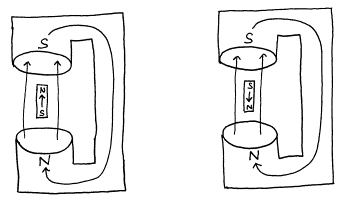
Figure 4. Low energy and high energy configuration of a magnet in an external field.
The energies of the above two cases may be calculated to be
Elow = -m B0 (14)
and
Ehigh = m B0 (15)
where m is the magnetic moment and B0 is the external magnetic field strength.
The energy of a magnet at an arbitrary orientation between these limits is calculated by multiplying the projection of the magnetic moment along the field direction by the field strength. Assuming that the quantization axis is taken to be the direction of the magnetic field, then the energy of the mI nuclear spin state is
![]() (16)
(16)
from which it is seen that the energies of different mI states of a nucleus are not degenerate in an external magnetic field. Hence, the m quantum number is called the magnetic quantum number.
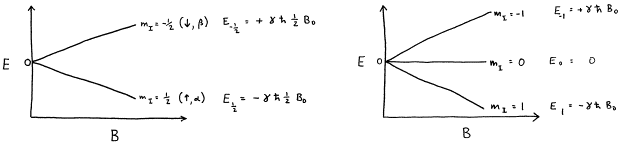
Figure 5. Energy levels for I=1/2 and I=1 as a function of magnetic field strength. (The relative scale of the diagrams is arbitrary, as different nuclear species have different values of g.)
Exercise 6: Calculate E for a mI=1/2 proton in a 1.41 T field. (Hint: T = V·s·m-2)
3.2 Boltzmann Distribution
In the presence of an external magnetic field, different mI nuclear spin states have different energies. At thermal equilibrium, they will also have different populations according to the Boltzmann equation
![]() (17)
(17)
where Nhigh and Nlow are the populations of the upper and lower states respectively, DE = Ehigh - Elow is the energy difference between the two states, k is the Boltzmann constant, and T is the absolute temperature.
Exercise 7: Calculate Nhigh/Nlow for protons in a 1.41 T field at 298 K. Calculate Nhigh/Nlow in a 7.05 T field. State the dependence of Nhigh/Nlow on magnetic field strength.
In the limit as T approaches zero (or DE approaches infinity), Nhigh/Nlow approaches zero implying that only Nlow is populated. In the limit as T approaches infinity (or DE approaches zero), Nhigh/Nlow approaches unity implying that Nlow and Nhigh are equally populated. In practice, the difference between nuclear spin energy levels DE in achievable fields is much smaller than kT, implying that Nlow is only very slightly in excess of Nhigh.
An analogy to the thermal population of Nlow and Nhigh is a collection of compasses which lie on a table. When the table is undisturbed, all the compasses will be in their low energy state and point toward the earth’s north pole. However, if the table is shaken, then some of the compasses will adopt the higher energy state of pointing toward the south pole. In practice, the thermal energy of shaking vastly exceeds the magnetic force attempting to align the magnets along the earth’s field, resulting in near equal numbers of compasses pointing north and south. Note that the shaking causes the compasses to continually switch directions that they are pointing. Thus the equilibrium between high energy and low energy configurations is a dynamic equilibrium, not a static equilibrium.
Exercise 8: Evaluate the “high temperature approximation” by calculating
![]() (18)
(18)
for protons in a 1.41 T field at 298 K and comparing it to the previously calculated exact answer.
3.3 Transition Frequencies
NMR spectroscopy is performed by inducing transitions between adjacent nuclear spin energy states (DmI = ±1). The energy change for a nucleus undergoing an NMR transition is
Equation (19) may be interpreted as
follows. The difference between angular
momentum z‑component
of adjacent mI
states is ![]() . This
difference is multiplied by g to obtain the difference in the
magnetic moment z‑component.
This result is then multiplied by the magnetic field strength to obtain the
energy difference between adjacent mI states in a magnetic field.
. This
difference is multiplied by g to obtain the difference in the
magnetic moment z‑component.
This result is then multiplied by the magnetic field strength to obtain the
energy difference between adjacent mI states in a magnetic field.
Exercise 9: Calculate DE for a proton in a 1.41 T field.
The frequency n of the electromagnetic radiation used to induce a NMR transition between adjacent mI levels in external magnetic field B0 is calculated from
DE = hn (20)
which yields
n = DE/h = g![]() B0/h = gB0/2p (21)
B0/h = gB0/2p (21)
The units of frequency (n) are cycles/second, which are also called Hertz (Hz). In NMR spectroscopy, it is often more convenient to use angular frequency (w) with units of radians/second. Since one cycle equals 2p radians,
w º 2pn (22)
Since cycles and radians are not SI units, both n and w have the same SI units (s-1). Thus, frequency (cycles/second) and angular frequency (radians/second) must be distinguished through careful use of the symbols of n and w, respectively. The angular frequency of an NMR transition is more commonly written as
which is the famous Larmor equation. Note that the use of w eliminates the occurrence of 2p in the Larmor equation.
Exercise 10: Calculate n and w for a proton in a 1.41 T field. In what region of the electromagnetic spectrum does this frequency occur? State why a NMR spectrometer with a 1.41 T magnet is commonly referred to as a “60 MHz spectrometer.”
4. Generation of the NMR Signal
4.1 Overview
The measurement of a NMR spectrum signal is very different from infrared or UV‑VIS spectroscopy in which absorption of photons is measured as a function of frequency. It has been argued that NMR spectroscopy is similar to emission spectroscopy, in that absorption of radiowave frequencies is detected indirectly by the subsequent emission of radiowaves at the same frequency. While this explanation can account for a simple proton NMR spectrum, it does not readily explain pulsed experiments that have give NMR its tremendous ability to uncover couplings and correlations among nuclei in a molecule. Furthermore, counterarguments have been made that the small NMR probe would be an very poor emitter and receiver of long wavelength radiofrequency radiation and the relatively low radiofrequency implies extremely low absorption and emission probabilities. Hence, it is better to view NMR spectroscopy as a magnetic induction experiment rather than as an experiment involving absorption and emission of photons.
A more complete understanding of NMR spectroscopy is achieved if one considers the effect of applied magnetic fields on the nuclear magnetic moments in the system. Briefly, an NMR sample in an external magnetic field is disturbed from equilibrium by application of a transverse secondary magnetic field, and its response to the disturbance is recorded. Furthermore, a tremendous enhancement of signal-to-noise, as well as the ability to carry out multiple-pulse experiments, occurs when a pulse of radiowave frequencies is used to disturb the system from equilibrium. The time dependence of the return to equilibrium is then measured, from which frequency spectrum is generated via a Fourier transform. In this section the underlying physical basis if the NMR phenomena is described. The implementation details in a modern spectrometer are covered in a subsequent section.
4.2 Net Magnetization Vector
It will be necessary to
distinguish between motion relative to the laboratory in which the external
magnetic field originates and motion relative to other coordinate systems. The cartesian coordinates associated with
the laboratory will be denoted X, Y, and Z.
If the magnetic moment vectors of a collection of nuclei are quantized
in the direction of the external magnetic field, then they have well-defined
projections along the Z‑axis but are randomly distributed
in the XY‑plane. Since there is a slight excess of the lower
energy projections aligned parallel to the field over the anti-parallel
projections, there is a slight net magnetization in the Z direction. However, there is no preference for the X
or Y
direction so there are equal numbers of projections onto this plane pointing in
each direction, resulting in no net magnetization in the XY‑plane. The net magnetization vector is labeled ![]() , and may also called the macroscopic magnetization vector or the equilibrium
magnetization vector.
, and may also called the macroscopic magnetization vector or the equilibrium
magnetization vector.
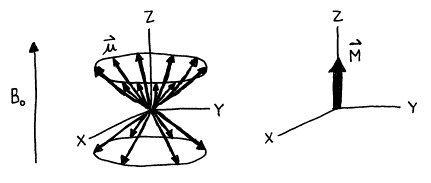
Figure 6. The vector sum of a collection of I=1/2 nuclear magnetic moments in an external magnetic field.
The measurement of the net magnetization vector was a very challenging task due to the small magnitude of the vector and the very large applied field in the same direction. Bloch and Purcell independently made this measurement for the proton in 1946, for which they were jointly awarded the Nobel Prize in physics. However, neither of them would have predicted that organic chemistry students would routinely make such measurements in order to distinguish among the same nuclear species in different chemical environments with differences in magnetic moments of less than 0.1 ppm!
Exercise 11: Calculate the net magnetization of 0.1 mL of water in a 1.41 T magnet at 298K. Compare this value to the earth’s magnetic field strength of approximately 1 gauss. (Hints: Each water molecule contains 2 H atoms, and O has an even number of protons and neutrons. 104 gauss = 1 T.)
4.3 Larmor Precession
When a rapidly spinning gyroscope is tipped from vertical, it does not fall over but rather experiences a torque given byand precesses about the earth’s gravitational field. Careful observation of a gyroscope’s precessional motion reveals that the precessional frequency is independent of tip angle and the tip angle remains constant during precession.
When a magnetic moment lies at an angle to an external magnetic field, it experiences a similar torque
![]() (24)
(24)
and it precesses about the external field. The precession frequency is given by the famous Larmor equation
w0 = gB0 (23)
and w0 is the Larmor frequency and B0 is the external magnetic field strength. The Larmor frequency has two important physical interpretations. It is the precessional frequency of the nuclear magnetic moment about the magnetic field. It is also the frequency of the electromagnetic radiation that induces a transition between nuclear spin quantum states in the magnetic field.
Since each individual nuclear
magnetic moment vector is tilted from the external magnetic field, it
experiences precessional motion about the Z‑axis. However, as long as the net magnetization vector ![]() lies along the Z‑axis,
it does not undergo precession.
lies along the Z‑axis,
it does not undergo precession.
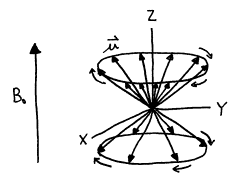
Figure 7. Precession of I=1/2 magnetic moment vectors in applied magnetic field B0.
4.4 Transverse Magnetic Field
Larmor precession of the
magnetization vector ![]() is not observed as
long as
is not observed as
long as ![]() lies in the direction
of the magnetic field
lies in the direction
of the magnetic field ![]() . In order to observe
precession of
. In order to observe
precession of ![]() about
about ![]() ,
, ![]() must be tipped away from the Z‑axis. This can be accomplished by introducing
another magnetic field
must be tipped away from the Z‑axis. This can be accomplished by introducing
another magnetic field ![]() that is perpendicular
to the Z‑axis.
that is perpendicular
to the Z‑axis.
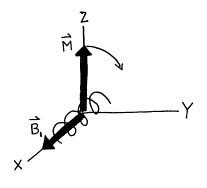
Figure 8. Coils in a NMR spectrometer which tip the magnetization vector M.
In a NMR spectrometer, the ![]() field is generated
through the use of a solenoid coil of wire whose axis lies in the XY‑plane. We will define the X‑axis to lie along
the axis of the coil. If electric current flows through the coils, a magnetic
field is generated along the X‑axis, and the magnetic moment is
tipped off the Z‑axis toward Y‑axis in accordance with Eq. (24). If
field is generated
through the use of a solenoid coil of wire whose axis lies in the XY‑plane. We will define the X‑axis to lie along
the axis of the coil. If electric current flows through the coils, a magnetic
field is generated along the X‑axis, and the magnetic moment is
tipped off the Z‑axis toward Y‑axis in accordance with Eq. (24). If ![]() were a static field,
then
were a static field,
then ![]() would precess about
the new overall magnetic field
would precess about
the new overall magnetic field ![]() at the Larmor
frequency. In an NMR spectrometer,
at the Larmor
frequency. In an NMR spectrometer, ![]() <<
<<![]() ; hence, the tip angle of
; hence, the tip angle of ![]() would be minimal and
a negligibly small NMR signal would be generated. Furthermore, relaxation effects would ultimately cause
would be minimal and
a negligibly small NMR signal would be generated. Furthermore, relaxation effects would ultimately cause ![]() to align with the new
to align with the new
![]() field, and precession
would stop. In short, use of a static
field, and precession
would stop. In short, use of a static ![]() field would result in
a very uninteresting NMR experiment, as it is equivalent to simply changing the
direction of the external magnetic field.
field would result in
a very uninteresting NMR experiment, as it is equivalent to simply changing the
direction of the external magnetic field.
A very different situation
arises if ![]() is not static but is
made to rotate with about the Z‑axis with the Larmor frequency w0. Then
is not static but is
made to rotate with about the Z‑axis with the Larmor frequency w0. Then ![]() continues to tip away
from the Z‑axis
toward the XY‑plane
so as to precess about the rotating
continues to tip away
from the Z‑axis
toward the XY‑plane
so as to precess about the rotating ![]() field at Larmor
frequency w1. The overall motion of
field at Larmor
frequency w1. The overall motion of ![]() in this case is quite
complex.
in this case is quite
complex. ![]() is a spiral motion on
the surface of a sphere, rotating about the Z‑axis at w0 and away from the Z‑axis
at w1. In this arrangement, the tip angle of
is a spiral motion on
the surface of a sphere, rotating about the Z‑axis at w0 and away from the Z‑axis
at w1. In this arrangement, the tip angle of ![]() away from the Z‑axis
can reach a full 90° and thereby
generate a significant NMR signal.
away from the Z‑axis
can reach a full 90° and thereby
generate a significant NMR signal.

Figure 9. Precession of ![]() about
about ![]() for stationary
for stationary ![]() and for rotating
and for rotating ![]() .
.
A rotating magnetic field is
generated by connecting the coils to a radiofrequency source whose output
voltage varies sinusoidally at frequency w0. An alternating current (AC) will flow in the
coil, producing a linearly oscillating magnetic field. The resulting magnetic field is colinear
with the X‑axis
but varies in magnitude so as increase along the positive X‑axis, reach a
maximum, decrease toward zero, increase along the negative X‑axis, reach a
maximum in this direction, and return to zero.
A linearly oscillating vector can be decomposed into the sum of two
counter rotating vectors. Each rotating
vector rotates at frequency w0;
however, one vector rotates in the same direction of Larmor precession of ![]() and the other in the
opposite direction. The component of
the magnetic field rotating with
and the other in the
opposite direction. The component of
the magnetic field rotating with ![]() is the
is the ![]() field. (It turns out that the other component has a
negligible effect on the net magnetization vector, as it differs too much in
frequency from w0.)
field. (It turns out that the other component has a
negligible effect on the net magnetization vector, as it differs too much in
frequency from w0.)
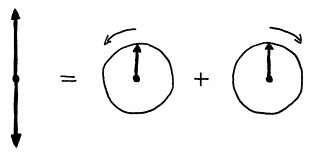
Figure 10. Decomposition of a linearly oscillating magnetic field into two counter rotating magnetic fields.
4.5 Rotating Frame of Reference
In order to simplify the motions of the nuclear magnetic moments during an NMR experiment, a new axis system is introduced which rotates in the same direction and rate as the moments are precessing. The axis system is called the rotating frame, and the axes are labeled with lowercase x, y, and z. Recall that the stationary or laboratory frame axes are labeled with uppercase X, Y, and Z. (Older literature, however, uses x, y, and z for the stationary frame and x', y', and z' for the rotating frame.) Use of the rotating frame allows one to visualize more easily all motion of the magnetic moments other than Larmor precession. An analogy to understand this is to consider reading the label on a record while it is being played on a turntable. It is difficult to read the label while you are stationary and the label is rapidly rotating; however, it would be much easier to read the label if you “jumped aboard” the rotating album and rotated with it, as the label would appear to not move in your new rotating frame. However, note that the label could appear upright, upsidedown, or even sideways, depending on when one jumped aboard the rotating frame.
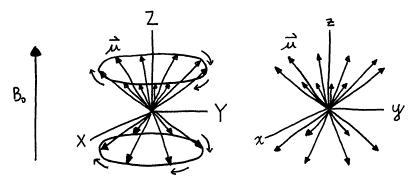
Figure 11. Rotational motion in the laboratory frame appears to stop in the stationary frame.
The motion of the rotating ![]() field is quite simple
in the rotating frame. Since the
rotating frame rotates at exactly the frequency of the
field is quite simple
in the rotating frame. Since the
rotating frame rotates at exactly the frequency of the ![]() vector , the
vector , the ![]() vector is a
stationary in the xy‑plane. The
motion of the magnetization vector in the rotating field is also very
simple. The precessional motion of
vector is a
stationary in the xy‑plane. The
motion of the magnetization vector in the rotating field is also very
simple. The precessional motion of ![]() about the z‑axis
at Larmor frequency w0 stops, and
about the z‑axis
at Larmor frequency w0 stops, and ![]() simply precesses
about the stationary
simply precesses
about the stationary ![]() vector at frequency w1.
vector at frequency w1.
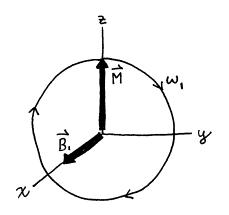
Figure 12. Motion of
magnetization vector ![]() in rotating frame of
reference.
in rotating frame of
reference.
It is quite clear in the
rotating frame system that the rotation frequency of ![]() must match the Larmor
frequency w0
in order to significantly tip the magnetization vector. Imagine that the
must match the Larmor
frequency w0
in order to significantly tip the magnetization vector. Imagine that the ![]() vector were rotating
slightly slower (or faster) than
vector were rotating
slightly slower (or faster) than ![]() which rotates at the
Larmor frequency w0. Initially, the
which rotates at the
Larmor frequency w0. Initially, the ![]() field would tip the
magnetization vector
field would tip the
magnetization vector ![]() away from the z‑axis
in a particular direction. However, the
mismatch between rotation frequency of
away from the z‑axis
in a particular direction. However, the
mismatch between rotation frequency of ![]() and the Larmor
frequency w0
would soon cause
and the Larmor
frequency w0
would soon cause ![]() to precess around the
z‑axis
to the side opposite of its initial tip.
The
to precess around the
z‑axis
to the side opposite of its initial tip.
The ![]() field would then tip
field would then tip ![]() back toward the z‑axis
restoring it to its original alignment.
The
only frequency at which the rotating magnetic field
back toward the z‑axis
restoring it to its original alignment.
The
only frequency at which the rotating magnetic field ![]() can significantly tip
the magnetization vector
can significantly tip
the magnetization vector ![]() is the Larmor
frequency w0. Thus, it is an excellent approximation to
ignore the component of the linearly oscillating magnetic field that rotates in
the opposite sense as
is the Larmor
frequency w0. Thus, it is an excellent approximation to
ignore the component of the linearly oscillating magnetic field that rotates in
the opposite sense as ![]() , for its rotational frequency is -2w0 in the rotating frame
and its effect on
, for its rotational frequency is -2w0 in the rotating frame
and its effect on ![]() is therefore
neglible.
is therefore
neglible.
Rotating the axis system at the Larmor precession frequency w0 is extremely useful. While Larmor precession is a consequence of the external magnetic field that is necessary to generate a NMR signal, it contains no useful information beyond the value of the magnetogyric constant g. Rather, all the interesting information in a NMR spectrum arises from the slightly different magnetic fields that nuclei experience due local environment differences, and this information is contained in the small differences among their Larmor frequencies rather than the total magnitude of the Larmor frequency. It is always easier to see a small signal when it is not on top of a large background. Hence, it is both mathematically convenient and experimentally necessary to reference all NMR signals to the Larmor frequency of the external magnetic field.
If ![]() is chosen to lie on
the x‑axis,
then
is chosen to lie on
the x‑axis,
then ![]() tips from the z‑axis
toward to y‑axis
according to Eq. (24). Note that because this is precessional
motion, the angle between
tips from the z‑axis
toward to y‑axis
according to Eq. (24). Note that because this is precessional
motion, the angle between ![]() and
and ![]() (90° in this case) is maintained (motion remains in
the yz‑plane
in this case). In a pulsed Fourier
Transform NMR spectrometer, is it possible to control the direction in which
(90° in this case) is maintained (motion remains in
the yz‑plane
in this case). In a pulsed Fourier
Transform NMR spectrometer, is it possible to control the direction in which ![]() tips, the rate at
which
tips, the rate at
which ![]() tips, and the amount
of the tip angle. The
tips, and the amount
of the tip angle. The ![]() field is generated by
a short pulse of radiofrequency (RF) into the coils which surround the sample
in the probe. The direction of tipping
is controlled by the relative phase between the applied radiofrequency pulse
and the rotating frame. For example, if
the maximum RF intensity occurs when rotating the x‑axis lies along the
coil, then the
field is generated by
a short pulse of radiofrequency (RF) into the coils which surround the sample
in the probe. The direction of tipping
is controlled by the relative phase between the applied radiofrequency pulse
and the rotating frame. For example, if
the maximum RF intensity occurs when rotating the x‑axis lies along the
coil, then the ![]() field is along the x‑axis. However, if the maximum RF intensity occurs
when the y‑axis
lies along the coil, then the
field is along the x‑axis. However, if the maximum RF intensity occurs
when the y‑axis
lies along the coil, then the ![]() field is along the y‑axis. The rate and magnitude of the tip is
determined by the RF power and the duration of the RF pulse, respectively. Stronger RF power creates a stronger
field is along the y‑axis. The rate and magnitude of the tip is
determined by the RF power and the duration of the RF pulse, respectively. Stronger RF power creates a stronger ![]() field that results in
a higher w1, and a longer RF pulse
causes the precession about
field that results in
a higher w1, and a longer RF pulse
causes the precession about ![]() precession to
continue further. It is the ability to
precisely control the tip of the magnetization vector
precession to
continue further. It is the ability to
precisely control the tip of the magnetization vector ![]() that makes multiple
pulse NMR experiments possible.
that makes multiple
pulse NMR experiments possible.
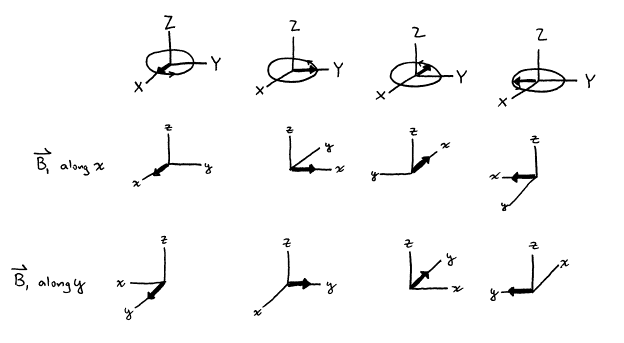
Figure 13. Generation of ![]() along the x‑axis
and along the y‑axis.
along the x‑axis
and along the y‑axis.
Current convention defines an “x‑pulse”
as a pulse that induces rotational of ![]() about the x‑axis
in a positive or Right Hand Rule sense, i.e., y®z®-y®-z. However, the older literature and many
textbooks define an x‑pulse in the opposite sense. As long as either convention is used
consistently, it will predict the same result for NMR experiments as the other
convention. Since both conventions are
widely found, one should not worry about the particular choice made an author
but should note the choice and follow it in that article or book. Careful consideration of Eq. (24)
and the definition of positive rotation reveals that
about the x‑axis
in a positive or Right Hand Rule sense, i.e., y®z®-y®-z. However, the older literature and many
textbooks define an x‑pulse in the opposite sense. As long as either convention is used
consistently, it will predict the same result for NMR experiments as the other
convention. Since both conventions are
widely found, one should not worry about the particular choice made an author
but should note the choice and follow it in that article or book. Careful consideration of Eq. (24)
and the definition of positive rotation reveals that ![]() . Thus, the magnetic
field which induces an x‑pulse is actually aligned along
the negative x‑axis for a nucleus with a positive magnetogyric
ratio. The choice of whether to define
an x‑pulse
with respect to the rotation vector or the magnetic vector is matter of
preference. However, current convention
is to focus attention on rotation of the magnetization vector rather than on
the radiofrequency magnetic field vector.
This is known as the Right Hand Convention, because if one aligns the
thumb of the right hand along the axis of the pulse, the fingers curl in the
direction that
. Thus, the magnetic
field which induces an x‑pulse is actually aligned along
the negative x‑axis for a nucleus with a positive magnetogyric
ratio. The choice of whether to define
an x‑pulse
with respect to the rotation vector or the magnetic vector is matter of
preference. However, current convention
is to focus attention on rotation of the magnetization vector rather than on
the radiofrequency magnetic field vector.
This is known as the Right Hand Convention, because if one aligns the
thumb of the right hand along the axis of the pulse, the fingers curl in the
direction that ![]() rotates.
rotates.
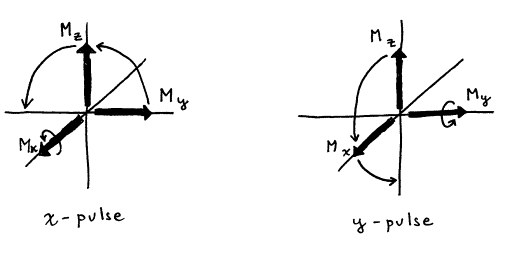
Figure 14. The effect of an x‑pulse and a y‑pulse
on the magnetization vector components
Exercise 12: What is n1
for a 10 ms pulse that rotates ![]() by 90° for a
proton? What field strength
by 90° for a
proton? What field strength ![]() is required for this
pulse? Compare the strength of
is required for this
pulse? Compare the strength of ![]() to
to ![]() for this case in a
300 MHz NMR spectrometer?
[Answers: 25kHz; 5.87 gauss;
12000:1]
for this case in a
300 MHz NMR spectrometer?
[Answers: 25kHz; 5.87 gauss;
12000:1]
Exercise 13: A typical RF coil in a probe consists of 2 turns of wire with 1 cm radius separated by 1 cm and has an impedance of 50 ohms. How much power must be delivered to the probe to produce the above 90 degree pulse? (Hints: A general physics textbook calculation reveals 1 A of current in a 1 loop coil with 1 cm radius produces a 6´10-5 T magnetic field. P=VI=I2R.)
We will later see that the rotating frame is more than a mathematical construct to assist in the visualization of motion of nuclear magnet moments. The rotating frame is actually the reference signal to the Phase Sensitive Detector in the NMR spectrometer!
4.6 Observation of the NMR Signal
Just as an oscillating electric
field in a coil of wire creates an oscillating magnetic field, an oscillating
magnetic field causes an oscillating electric field in the wire. The alternator found in every automobile
operates on this principle. The NMR
signal is observed with a coil in the XY‑plane similar to the transmitter
coil that detects the rotating magnetization vector ![]() . Early NMR
spectrometer designs used separate transmitter and receiver coils; however,
modern designs use a single transceiver coil that is electronically
connected to the transmitter during the pulse and then to the receiver after
the pulse. Although
. Early NMR
spectrometer designs used separate transmitter and receiver coils; however,
modern designs use a single transceiver coil that is electronically
connected to the transmitter during the pulse and then to the receiver after
the pulse. Although ![]() is stationary in the
rotating frame, it is moving at the Larmor frequency in the stationary
frame. As
is stationary in the
rotating frame, it is moving at the Larmor frequency in the stationary
frame. As ![]() , the projection of
, the projection of ![]() on the xy‑plane,
cuts through the turns of the received coil, it induces a RF current at
frequency w0. The spectrometer looks for an electrical
signal at w0
in the receiver coil, typically by comparing the output signal to the input
signal with a Phase Sensitive Detector as discussed in a subsequent section.
on the xy‑plane,
cuts through the turns of the received coil, it induces a RF current at
frequency w0. The spectrometer looks for an electrical
signal at w0
in the receiver coil, typically by comparing the output signal to the input
signal with a Phase Sensitive Detector as discussed in a subsequent section.
Observation of the NMR signal
requires that the magnetization vector be tipped toward the xy‑plane. This tipping only occurs when the transverse
field ![]() rotates at the same
frequency as the Larmor precession frequency w0, i.e.,
when the RF transmitter frequency is tuned to the Larmor frequency. The transfer of RF electrical energy into
tipping of the magnetization vector relies on a close match between the applied
RF frequency and Larmor precession frequency.
The physical phenomenon of two oscillating systems transferring energy
most efficiently when their frequencies are the same is called resonance. Hence the name nuclear magnetic resonance
is a very appropriate term to describe the frequency dependence of coupling RF
electrical signal into the tipping of the magnetic moment vector.
rotates at the same
frequency as the Larmor precession frequency w0, i.e.,
when the RF transmitter frequency is tuned to the Larmor frequency. The transfer of RF electrical energy into
tipping of the magnetization vector relies on a close match between the applied
RF frequency and Larmor precession frequency.
The physical phenomenon of two oscillating systems transferring energy
most efficiently when their frequencies are the same is called resonance. Hence the name nuclear magnetic resonance
is a very appropriate term to describe the frequency dependence of coupling RF
electrical signal into the tipping of the magnetic moment vector.
Optional Exercise 14: Estimate the NMR signal power (in watts) of a 0.1 mL water sample in a 1.41 T magnet (exercise 11) observed with a typical RF probe (exercise 13).
4.7 Relaxation
The relaxation of net
magnetization vector ![]() has been ignored
until now. Recall that the equilibrium
which produces
has been ignored
until now. Recall that the equilibrium
which produces ![]() is a dynamic
equilibrium and that thermal energy constantly causes transitions between
nuclear spin states. The equilibrium
population among the nuclear spins states is determined by the Boltzmann
distribution. When the system is
perturbed from equilibrium by application of
is a dynamic
equilibrium and that thermal energy constantly causes transitions between
nuclear spin states. The equilibrium
population among the nuclear spins states is determined by the Boltzmann
distribution. When the system is
perturbed from equilibrium by application of ![]() , it will relax back toward equilibrium. The specific mechanisms for relaxation are
quite complex and will be discussed in a later section. Factors that affect the relaxation rate
include nuclear species, magnetic field homogeneity, temperature, and presence
of magnetic material (ranging from other nuclei with spin to paramagnetic
species such as a dilute aqueous CuSO4 solution).
, it will relax back toward equilibrium. The specific mechanisms for relaxation are
quite complex and will be discussed in a later section. Factors that affect the relaxation rate
include nuclear species, magnetic field homogeneity, temperature, and presence
of magnetic material (ranging from other nuclei with spin to paramagnetic
species such as a dilute aqueous CuSO4 solution).
If ![]() is tipped by a short
pulse of radiofrequency, it will commonly take several seconds for
is tipped by a short
pulse of radiofrequency, it will commonly take several seconds for ![]() to return to its
equilibrium position along the z‑axis. Two distinct kinds of relaxation occur, each with its own time
constant. Relaxation of
to return to its
equilibrium position along the z‑axis. Two distinct kinds of relaxation occur, each with its own time
constant. Relaxation of ![]() along the z‑axis
occurs with time constant T1, which typically varies from seconds for protons to tens of
seconds for carbon. Since T1
is a measure of the time required for magnetization vector to return to its
equilibrium length and direction, it directly affects when the next
radiofrequency pulse can be applied.
The delay before applying the next pulse can vary from zero to 5 T1,
depending on the specific experiment being carried out. Relaxation of the vector magnitude in the xy‑plane
occurs with time constant T2*, which is typically on the
order of a second or two. Since T2*
is a measure of the time required for the magnetization vector to leave the xy‑plane,
it is a direct measure of the length during which a signal can be
observed. The magnitude of T2*
therefore affects the length for which a FID should be recorded. A general guideline is to collect the FID
for 3 to 4 T2*.
along the z‑axis
occurs with time constant T1, which typically varies from seconds for protons to tens of
seconds for carbon. Since T1
is a measure of the time required for magnetization vector to return to its
equilibrium length and direction, it directly affects when the next
radiofrequency pulse can be applied.
The delay before applying the next pulse can vary from zero to 5 T1,
depending on the specific experiment being carried out. Relaxation of the vector magnitude in the xy‑plane
occurs with time constant T2*, which is typically on the
order of a second or two. Since T2*
is a measure of the time required for the magnetization vector to leave the xy‑plane,
it is a direct measure of the length during which a signal can be
observed. The magnitude of T2*
therefore affects the length for which a FID should be recorded. A general guideline is to collect the FID
for 3 to 4 T2*.
4.8 Factors Affecting Sensitivity
The NMR signal depends linearly
on the magnitude of magnetization in the xy‑plane,
![]() . This quantity is
governed by four factors for a given nucleus.
First, the tip angle of the magnetization vector affects
. This quantity is
governed by four factors for a given nucleus.
First, the tip angle of the magnetization vector affects ![]() . The closer the tip
angle is to 90°, the larger the magnitude of the magnitization vector is in the
xy‑plane. Second, the external magnetic field strength
affects the magnitude the magnetization vector
. The closer the tip
angle is to 90°, the larger the magnitude of the magnitization vector is in the
xy‑plane. Second, the external magnetic field strength
affects the magnitude the magnetization vector ![]() , and consequently its projection in the xy‑plane. Third, the sample temperature also affects magnetization vector
, and consequently its projection in the xy‑plane. Third, the sample temperature also affects magnetization vector ![]() , as a colder sample results in a larger population
difference between spin states. Fourth,
the number of nuclei in the coil, which is usually controlled by sample
concentration, also directly affects the magnetization vector
, as a colder sample results in a larger population
difference between spin states. Fourth,
the number of nuclei in the coil, which is usually controlled by sample
concentration, also directly affects the magnetization vector ![]() .
.
The NMR signal also depends on the magnetogyric ratio g raised to the third power. This strong dependence arises from the magnetic moment depending linearly on g, the population difference between spin states depending linearly on g, and the efficiency of signal generation depending on the Larmor frequency w, which is proportional to g.
Exercise 15: Estimate the relative sensitivity of 1H and 13C NMR spectra. (Hint: account for relative abundance, relaxation, and magnetogyric ratio.)
5.1 Different Nuclei (magnetogyric ratio)
According to the Larmor equation, the precessional frequency of a nuclear magnetic moment is proportional to the magnetogyric ratio and the magnet strength.
w0 = gB0 (23)
Different nuclei have different magnetogyric ratios (see Table 2). Thus for a given magnetic strength, the frequency at which nuclei of different elements precess depends on their identity and differs vastly.
It is common practice to characterize magnet strength by a precessional frequency, e.g., a 300 MHz magnet. Such terminology assumes a specific nucleus, typically a proton. Thus in a 300 MHz spectrometer (for protons), the precessional frequency of carbon is 75 MHz.
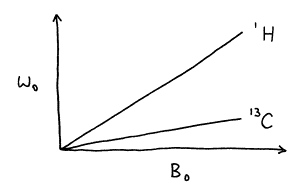
Figure 15. Dependence of precessional frequency on magnet strength on the magnetogyric ratio.
5.2 Inequivalent Nuclei (shielding)
All atoms in a molecule are
surrounded by electrons that occupy core and valence orbitals. The permanent magnetic field ![]() induces a current in
the surrounding electrons, which in turn generates an induced magnetic field
induces a current in
the surrounding electrons, which in turn generates an induced magnetic field ![]() . According to Lenz’s
Law, the induced field is proportional to the permanent magnetic field but is
opposite in direction
. According to Lenz’s
Law, the induced field is proportional to the permanent magnetic field but is
opposite in direction
![]() = -s
= -s![]() (25)
(25)
where the proportionality factor s is called the shielding constant.
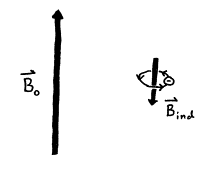
Figure 16. Electrical current flow from
surrounding electrons induces ![]() to oppose
to oppose ![]() .
.
The local magnetic field experienced by a nucleus is the sum of the permanent field and the induced field
![]() =
= ![]() +
+ ![]() (26)
(26)
Nuclei in different chemical environments are called inequivalent nuclei, and they experience slightly different local fields and therefore precess at slightly different Larmor frequencies
w0 = g Bloc = g (B0 + Bind) = g (B0 - s B0) = g B0 (1 - s) (27)
Nuclear magnetic resonance frequencies are always reported with respect to a standard. The standard for protons and for carbon is tetramethylsilane (TMS), Si(CH3)4. If the frequency difference is reported in Hertz, then it is proportional the external field strength. Given nucleus A and standard B with Larmor frequencies wA and wB, the chemical shift of A with respect to B is
nA-nB = (wA-wB) / 2p = [g B0 (1- sA) / 2p] - [g B0 (1- sB) / 2p] = g B0 (sB- sA) / 2p (28)
Thus, the energy spacing between two inequivalent nuclei is proportional to magnetic field strength B0. This increase in spectral separation with field strength is one of the main advantages of using higher strength magnets in modern NMR spectrometers. (The other advantage is the higher sensitivity achieved due to the greater population difference between spin states.)
It would be quite inconvenient to report nuclear magnetic resonance frequency differences in Hertz because the strength of every magnetic is different. Dividing by the frequency of a standard, which is also dependent on field strength, eliminates this dependence on magnetic field strength.
![]() (29)
(29)
since sB<<1. The chemical shift in parts per million d of nucleus A is the usual quantity reported in the literature.
![]() (30)
(30)
As will be seen in future sections, however, it can also be useful to think about the chemical shift relative to the standard in Hertz, especially when considering Fourier Transform NMR and spin-spin coupling strengths.
In general, the amount of shielding is proportional to the local electron density, i.e., higher electron density causes more shielding and results a lower Larmor frequency. However, it is possible for some chemical groups with circular p electron systems, most notably aromatic rings and triple bonds, to cause induce chemical shifts which are not the same for all orientations in space, a phenomenon known as chemical shift anisotropy.
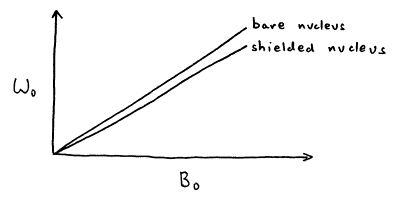
Figure 17. Shielded nucleus has a lower Larmor frequency.
Figure 18. Induced field ![]() can cause chemical
shift anisotropy.
can cause chemical
shift anisotropy.
Exercise 16: Consider the proton in chloroform, Cl3CH, and the chemically equivalent protons in tetramethylsilane (TMS), Si(CH3)4. Which proton has the higher Larmor frequency? Explain your reasoning. (Hint: Consider the electronegativity of the other atoms in each molecule.) [Answer: Chlorine is more electronegative than silicon. Thus, the proton in chloroform is less shielded and has a higher Larmor frequency.]
Exercise 17: The CHCl3 proton resonance is observed at 436 Hz in a 60 MHz spectrometer and at 2181 Hz in a 300 MHz spectrometer relative to TMS. Calculate the chemical shift d in ppm in each case. [Answer: 7.27 ppm in each case]
5.3 Equivalent Nuclei (spin-spin coupling)
Nuclei with spin can interact, or couple, with each other. Since each nucleus can be thought of as a small magnet, the orientation of that magnet has an effect on the local magnetic field experienced by other nuclei. This effect is most prominent among chemically equivalent nuclei, giving rise to the N+1 rule for equivalent protons learned in organic chemistry. A proton with N protons on contiguous carbon atoms splits into N+1 peaks with intensity pattern given by 1:1, 1:2:1, 1:3:3:1, ... for N equal to 1, 2, 3, ... Spin-spin coupling will be discussed in much greater detail in a future section, with extensions to more complicated interactions including interactions between different nuclei.
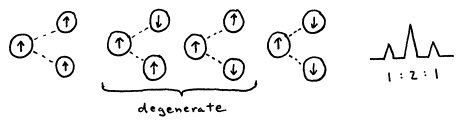
Figure 19. 1:2:1 intensity pattern arises from one proton coupling with two equivalent protons.
Exercise 18: Qualitatively predict the chemical shift and spin coupling pattern of the ethyl group, CH2CH3, in ethanol, HOCH2CH3. Justify your assignments.